Portions of the text below have been excerpted from the following NALMS publications:
Carlson, R.E. and J. Simpson. 1996. A Coordinator’s Guide to Volunteer Lake Monitoring Methods. North American Lake Management Society. 96 pp.
Introduction
Chlorophyll is the green molecule in plant cells that carries out the bulk of energy fixation in the process of photosynthesis. Besides its importance in photosynthesis, chlorophyll is probably the most-often used estimator of algal biomass in lakes and streams, at least in North America. Its popularity results from several considerations:
- it is a measure of algal biomass that is relatively unaffected by non-algal substances,
- it is a fairly accurate measure of algal weight and volume, and,
- it acts as an empirical link between nutrient concentration and a number of important biological phenomena in lakes and reservoirs.
Chlorophyll is also relatively easy to measure. This facility of measurement contributes to its popularity, but the resulting values are far more ambiguous than most are willing to admit.
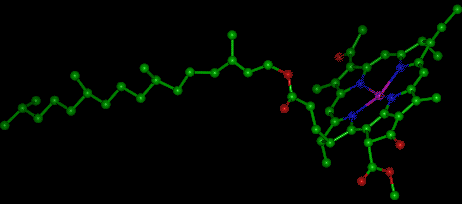
Chlorophyll itself is actually not a single molecule but a family of related molecules, designated chlorophyll a, b, c, and d. Chlorophyll a is the molecule found in all plant cells and therefore its concentration is what is reported during chlorophyll analysis. Chlorophyll d is found only in marine red algae, but chlorophylls b and c are common in fresh water. The molecular structure of chlorophylls a and b consists of a ring-like structure called a porphyrin and a long organic phytol “tail.” In the center of the porphyrin ring is a magnesium molecule (Fig. 1). Chlorophyll c lacks the phytol chain. The relative concentrations within the cell of these chlorophylls varies with the species of algae, but chlorophyll a is dominant in all the eukaryotic algae and the prokaryotic blue-green algae (Cyanobacteria).
Other pigments are also present in algal cells. These are the carotenes and the xanthophylls. In the cyanobacteria, water-soluble phycobiliproteins are the predominant accessory pigment, giving the group their characteristic blue-green or red color. In addition to the algal pigments, some bacteria are also pigmented with a series of bacteriochlorophylls.
In addition to the naturally occurring pigments in algal cells, a filtered water sample will also contain colored degradation products of these pigments. When algal chlorophyll degrades, it forms a series of degradation products, the nature of which depends on what part of the olecule that is affected. As a chlorophyll degrades, the initial step is either the loss of the magnesium from the center of the molecule or the loss of the phytol tail. The former pathway results in the formation of the molecule, phaeophytin; in the latter pathway, the resulting molecule is termed a chlorophyllide. The degradation scheme is shown in Figure 2. Further degradation of either the phaeophytin or the chlorophyllide produces a molecule termed a phaeophorbide: phaeophytin is degraded by the loss of the phytol tail and a chlorophyllide loses its magnesium ion. When a chlorophyll molecule breaks down, a number of distinct phaeophytins, chlorophyllides, and phaeophorbides will be produced, depending on the parent molecule. Some of these breakdown products.
| Figure 2. The degradation pathways of chlorophyll | 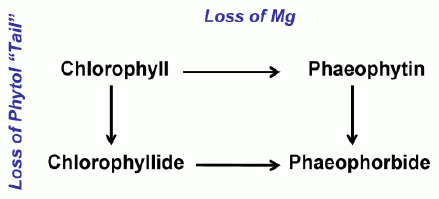 |
When a sample is filtered and extracted for chlorophyll analysis, it unfortunately contains a large number of these pigments other than chlorophyll a, the primary pigment of interest in monitoring programs. The absorbencies of these other pigments are not easily separable spectrophotometrically or fluorometrically from their parent molecule, producing falsely high absorbencies and subsequent erroneous values for chlorophyll a. Despite its seeming simplicity in the analysis of chlorophyll, the validity of its results depends on whether or not these interferences are adequately removed. Almost every choice of analytical method addresses specific interferences, yet ignores others. It therefore becomes the decision of the program coordinator to choose the analytical procedure that produces the most useful information for the program, not necessarily the most accurate estimate of chlorophyll a. It may be that some of the simpler techniques are more than adequate for the purposes of a monitoring program.
Field Techniques for Collection and Preservation of Chlorophyll Samples
Filtration
By some means, the volunteer must gather a sample of water, using either a hose sampler, some sort of water sampling bottle, or by simply lowering the sample container over the side of the boat. Once the sample is taken, it is usually filtered and preserved until delivered to the laboratory for analysis. An alternative to filtering, preservation, and storage would be to immediately deliver the whole water sample to the laboratory. Herve and Heinonen (1982) suggest that whole-water samples stored at 4°C in the dark can be kept up to 1 day without significant degradation of chlorophyll. Weber et al. (1986) found no change in refrigerated samples over 18 days, but if the samples were left at room temperature (20°C), 50% of the chlorophyll was lost in 5 days.
Filtration is usually accomplished in volunteer monitoring programs using a filtration funnel and hand-held suction pump. This system allows the volunteer to filter large amounts of water in a relatively short time. The volunteer can also see how much algae are being collected on the filter, and therefore judge when it is sufficiently green. This filtration technique does have the problem that the volunteer must measure the water in a separate, graduated container and must handle the filter, both before and after the filtration.
The Ohio-NEFCO program uses a 25 mm in-line Swinney filter holder instead of a filter funnel. The glass fiber filter is placed into the filter holder by the coordinating laboratory prior to distribution to the volunteers. The volunteers are given a 60 ml plastic syringe equipped with a latex rubber hose and a 3-way valve. After the sample is brought into the boat, the volunteer places the tube into the sampler, draws 50 ml of sample into the syringe, and pushes the sample gently through the filter, which is positioned on the output end of the 3-way valve. Pulling back on the plunger switches the valve back into input mode and a second 50 ml of sample is drawn up into the syringe. This alternation of drawing the sample and pushing it through the filter is done until an appropriate amount of algae have been filtered (See below). When finished, the volunteer puts the exit end of the filter holder on the input tube and draws any remaining water out of the sampler. The entire sampler is then wrapped in foil, labeled, and stored in the freezer until picked up for analysis. The volunteer never needs to touch or manipulate the filter.
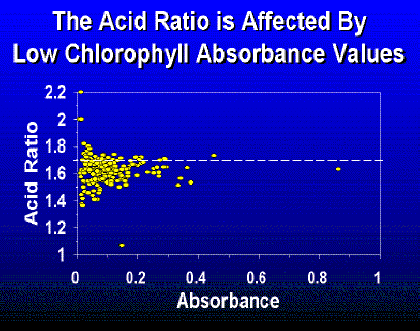
The amount algae filtered by the volunteer is important. There appears to be more variability in the acid ratio at low absorbencies (Fig. 3) that may contribute to error in chlorophyll a values. This variability may be a result of too much acidity causing phaeophytin to be degraded to phaeophorbide (Hallegraeff, 1976) or an analytical error associated with low chlorophyll concentrations. Standard Methods (APHA, 1989) recommends an optical density of 0.1 to 1.0 at 664 nm if the trichromatic method is used.
Some programs instruct the volunteer to continue filtering until the filter is slightly green. The Ohio-NEFCO and Wisconsin programs developed a relationship between Secchi transparency and the amount of water filtered (Table 1). The Ohio-NEFCO table works reasonably well except in reservoirs having high non-algal turbidity. In that case, the amount of algae filtered is insufficient. In Florida, the volunteer is instructed to filter a volume of approximately 100 milliliters for each foot of Secchi depth.
| Table 2. The relationship between Secchi depth and the amount of water filtered used in the Wisconsin Self-Help Lake Monitoring Program (Betz, et al. 1992) and in the Ohio-NEFCO program | |||
|
Wisconsin |
Ohio-NEFCO |
||
|
Secchi Depth (Ft) |
Volume Filtered (ml) |
Secchi Depth (Ft) |
Volume Filtered (ml) |
| < 1.0 | 50 | ||
| 1.0 – 1.5 | 100 | ||
| > 1.5 – 2.25 | 200 | < 2.0 | 100 |
| > 2.25 – 3.25 | 300 | ||
| > 3.25 – 6.0 | 500 | 2.1 – 4.0 | 200 |
| > 6.0 – 9.75 | 800 | 4.1 – 8.0 | 300 |
| > 9.75 – 16.5 | 1000 | > 8.0 | 400 |
| > 16.5 | 1500 | ||
Choice of Filters
There are differences of opinion as to what type of filter to use. Three factors have been considered: retention of particles, efficiency of extraction, and cost.
Membrane filters such as Millipore HA™ or Gelman™ retain more particles (Lenz and Fritsche, 1980), but are more subject to clogging than are glass fiber filters. This can mean that the volunteer might either have to filter less water or have to use very long filtration times. It also means that if a volunteer is faced with a clogged filter, they have to make a decision as to how to proceed: either start over, continue filtering, or, as is done in the Wisconsin program, pour the sample into another container, change the filter, and resume filtering.
Glass fiber filters have the advantage of being less expensive than membrane filters and, during grinding, the glass fibers aid in the homogenization of cells. The membrane filters can be ground in a tissue grinder, but not as efficiently as glass fiber filters. However, unless the superior extracting powers of a solvent such as methanol is used with membrane filters (see below), the advantages of superior retention of smaller particles by membrane filters may be lost because of a lower amount of extracted chlorophyll. Since Phinney and Yentsch (1985) suggest that major retention differences between membrane and glass fiber filters disappear above chlorophyll concentrations of 1 µg/L glass fiber filters would seem to be adequate for most inland waters. Prepas et al. (1988) found no difference in chlorophyll concentrations collected on either Whatman GF/F (median retention size of 0.7 µm) and GF/C (median retention size of approx 0.2 µm) glass fiber filters in concentrations ranging from 2 – 175 µg/L. In oligotrophic waters, the choice of filter may be of more concern.
Marker et al. (1980) listed the following reasons for using a Whatman GF/C glass fiber filter:
- They are very efficient for chlorophyll retention in most situations and sometime even better than membrane filters.
- They filter much faster and do not clog so rapidly.
- On centrifugation there is no turbidity in acetone or methanol extracts.
- They act as an excellent abrasive material to aid cell breakage during grinding.
- For comparisons between various chemical variables, it is essential that the filters used for the determinations should retain the same size fractions of particulate material, and most investigators now use glass-fiber filters for these determinations as well as for particulate organic carbon.
Some methods suggest the addition of MgCO3 to retard degradation and to enhance filtration efficiency. Some studies have found that its addition has no significant effect (Lenz and Fritsch 1980), and it may absorb pigments (Daley et al. 1973; Weber et al. 1986). If it is used with glass fiber filters, it should be used prior to filtration to decrease the pore size of the filter.
Preservation of the Chlorophylls
Once the chlorophylls are on the filter, they become highly susceptible to degradation as the cells die and decompose. They also become increasingly light and temperature labile. Some method must be used to keep the pigments from degrading. The problem is compounded for volunteer programs because the samples have to be transported to the laboratory for analysis. A simple mailing of the samples would be desirable, but there is the real possibility of degradation of the samples during the process.
The simplest method of preservation apparently is to freeze the samples. Several authors report that frozen samples showed no significant degradation even after 6 months (Lenz and Fritsche 1980). Jones and Lee (1982), however, mention that they have encountered problems with freezing and recommend that samples should not be frozen unless investigation has shown that results from frozen samples are comparable to those from fresh samples. A problem with freezing the filters is that apparently the chlorophyll will begin to degrade as soon as it is unfrozen. This means that the samples must be brought to the laboratory in a frozen state. This would seem to preclude the use of the mails to get the samples to the laboratory if they are not kept frozen during shipment.
Another preservation method is to immediately submerge the filter in the solvent, seal and darken. Apparently the chlorophyll will not degrade as long as it is kept dark. This means, however, that the volunteer would have to be given the solvents and that transportation would have to be of a liquid chemical.
Others have found that as long as the filter is kept dry and in the dark, the chlorophyll will not degrade. More experimentation seems to be necessary before an adequate preservation technique can be recommended.
Laboratory Analysis for Chlorophyll
Several methods for chlorophyll analysis are available. The methods are carefully described in Standard Methods (APHA 1991), and the methods will not be discussed in detail here. However, there is a great deal of confusion about which method should be used in limnological investigations, and this confusion has resulted in a number of different methods being used by various volunteer programs. Unfortunately, although all of these methods report their results as chlorophyll a, there is little evidence that the numbers derived by each method are necessarily similar. Because monitoring programs imply that the numbers generated are accurate as well as precise, the choice of a technique is important. A little background about chlorophyll analysis might help clarify the differences between the various chlorophyll methodologies used.
Choice of Solvents
Homogenization by grinding of the filter enhances the rupture of the algal cells and increases extraction efficiency of the solvent. Homogenization is an absolute necessity with an acetone solvent, but some have found that other extractants such as ethanol or methanol apparently do not need grinding to extract all the chlorophyll (Sartory and Grobbelaar, 1984). Others, however, have found that even methanol extractants do not extract as well without grinding. These other solvents are more efficient than acetone at extracting pigments from some green and blue-green algal cells. Methanol, however, is more toxic. Membrane filters can be ground but they lack the abrasiveness to produce a good extraction, and their extraction efficiencies are lowered (Long and Cooke 1971).
Measuring Chlorophyll
There are three basic methods for measuring chlorophyll.
Spectrophotometric Measurement
Spectrophotometric analysis of chlorophyll pigments were developed in the 1930s and 1940s (Weber et al. 1986). Richards and Thompson (1952) introduced a trichromatic technique that was supposed to measure chlorophylls a, b, and c. Trichromatic equations attempted to remove interferences of the other chlorophylls at the maximum absorption wavelength for each chlorophyll. Since Richards and Thompson, a number of modifications have been made to these equations which purportedly produce better estimates of the chlorophylls (Parsons and Strickland 1963; UNESCO 1966; Jeffrey and Humphrey 1975). When these equations are ultimately compared with concentrations of chlorophyll obtained using physical separation techniques such as HPLC, paper or thin-layer chromatography, it is found that the degree of correspondence is low. Apparently the trichromatic equations are no substitute for physical separation techniques. In addition, these equations do not deal with the degradation products of chlorophyll. The trichromatic “chlorophyll a” is better presented as chlorophyll a minus most of the interference of other chlorophylls but including all degradation products that have absorbencies at the primary wavelength of chlorophyll a. These multiple chlorophyll equations have not been particularly successful, but are still used in oceanographic research, where degradation products are less of a problem.
Lorenzen (1967) and Moss (1967) introduced an acidification step in a monochromatic method to circumvent the interference by chlorophyll degradation products. When chlorophylls are acidified, the magnesium ion is lost from the porphyrin ring, resulting in the production of a phaeophytin. Lorenzen (1967) produced equations capitalizing on the fact that the ratio of pure chlorophyll a after acidification to that before was 1.7. If the sample contained pure phaeophytin, then the absorbance would not change, and the ratio would be 1.0. Acid ratios between 1.0 and 1.7 would therefore indicate the amount of degradation products in the sample, and the estimate of chlorophyll could thus be corrected.
In natural waters, the acid ratio, and therefore the resulting estimate of chlorophyll a, varies considerably depending on, not only the relative concentrations of chlorophyll a and phaeophytin, but also the concentrations and behavior after acidification of chlorophylls b, phaeophorbides, chlorophyllides, bacteriochlorophylls, and perhaps phycobiliprotein pigments. Numerous modifications have been made to this technique, including changing the normality of the acid, the absorbance wavelengths, the time between acidification and the reading of the value, and the extracting solvent. Despite major modifications in the technique, the resultant value is always called chlorophyll a, even though each methodological change alters the estimated chlorophyll a value. For example, other pigments, particularly chlorophyllide a, cannot be spectrophotometrically distinguished from chlorophyll a. These magnesium-containing degradation products of chlorophyll can comprise a significant proportion of the total pigments. Hallegraeff (1976) found magnesium-containing degradation products, on some dates, to comprise over 50% of what would have been estimated as chlorophyll a. Hallegraeff (1976), as well as a number of other authors, emphasize that the acidification technique really eliminates only the interference of non-manganese-containing pigments. Remember also that the trichromatic technique supposedly produced more accurate chlorophyll a estimates by removing interferences from other chlorophylls; the phaeo-pigment technique does not correct for these interfering pigments, but will incorporate the change in absorption of these pigments when they degrade upon acidification.
Spectrophotometrically determined “chlorophyll a,” whether determined using trichromatic equations, or using the pigment correction is really an operationally defined term, whose meaning and values change with each change in the technique. Numerous authors emphatically state that the only method for measuring chlorophyll a accurately is using some separation procedure such as HPLC. Any other method produces only an estimate of the chlorophyll concentration.
An alternative to the use of physical separation techniques and the distress of choosing the “proper” spectrophotometric equation is to report the amount of total chlorophyll pigments (Golterman and Clymo 1971). It is the estimate of all chlorophyll pigments and degradation products that absorb at 665 nm. The measure is a descendent of the Odum’s et al. (1958) monochromatic chlorophyll a equation. Golterman and Clymo’s equation uses the extinction coefficients of Strickland and Parsons (1963) in 90% acetone which are probably the most popular extinction coefficients and solvent. Their equation is:
where V is the volume filtered (L), ν is the volume of extract (ml), and p is the pathlength (cm). Using values for total chlorophyll pigments rather than either the trichromatic equations or the acid-corrected equations gets around the problem of interference by ignoring it. It is simply a measure of absorbance at 665 nm.
There are some very good reasons for ignoring, or at least giving second place to, chlorophyll a values. Comparisons of the total chlorophyll concentration with trichromatic chlorophyll a calculated by the Parsons and Strickland (1963) equations using data from the Ohio-NEFCO program have correlations with the trichromatic chlorophylls of greater that 0.99 with a slope of 1. Herve and Heinonen (1982) also reported no significant differences between the Parsons and Strickland (1963) and the “Proposed Norsk Standard” chlorophyll equation, which is identical to that for total chlorophyll pigments. Canfield (personal communication) has not found sufficient amounts of phaeo-derivatives in Florida lakes to warrant reporting anything except total chlorophyll pigments. In most northern Ohio lakes, correlations between acid-corrected “chlorophyll a” and total chlorophyll have a correlation coefficient of 0.96 (n = 88). If, on most occasions, neither interference by other chlorophylls or by -derivatives significantly interfere with the chlorophyll a determination, the designation “chlorophyll a” could be used, but it is avoids confusion to use the term “total chlorophyll,” which implies no correction for chlorophyll or phaeo-derivatives but also does not perpetuate the myth that chlorophyll a can be accurately determined in natural water by spectrophotometric techniques.
Other Considerations for Spectrophotometric Determination of Chlorophyll
The bandwidth of the spectrophotometer is important because the wider the bandwidth, the lower the absorbance that is obtained (Weber, et al. (1986). This relationship results from the rather sharp chlorophyll peak. On instruments with large bandwidth, the value includes a greater amount of lower absorption values than would be obtained on an instrument with a narrow bandwidth. Standard Methods recommends using instruments with bandwidth of 0.5 – 2.0 nm.
If phaeo-pigments are to be determined spectrophotometrically, it is necessary to acidify the sample after the first reading. It is important to follow the Standard Methods procedures exactly. Any deviation of procedure at this step can produce the type of erroneous chlorophyll a results mentioned earlier. The amount of acid that has been used in the past has varied considerably. Lorentzen (1968) used several drops of 4M HCl in his original technique, but Riemann (1978) found that such a strong acid causes spectral shifts in the carotenoid, fucoxanthin, which increases in absorbance with acidification and therefore lowers the acid ratio. This shift also increases the value at 750 nm. A strong acid will also convert phaeophytin, and chlorophyllides to phaeophorbide, which results in an acid ratio greater than 1.7. This results in negative phaeo-pigment values and chlorophyll a values higher than total chlorophyll.
The amount of time between the addition of the acid and the reading of the absorbance is critical. The conversion from chlorophyll to phaeophytin is a first order reaction, the rate of which is dependent on pH. When strong acids were added, as used in the Lorenzen technique, conversion was instantaneous. The 90 seconds recommended in Standard Methods is necessary to complete most of the reaction, yet avoid the interference of degradation products of chlorophyll b, which increase at a much slower rate than those of chlorophyll a.
Fluorometric Techniques
When exposed to blue light, chlorophyll molecules will fluoresce brightly in the red region of the spectrum. Fluorometry is a highly sensitive method to determine chlorophyll concentration. This sensitivity can be of value in a volunteer monitoring program because the volunteer will not have to filter as much of a sample than is necessary in spectrophotometric analysis. Even multi-chromatic fluorescence equations exist (Loftus and Carpenter 1971).
Aside from sensitivity, however, there is little to recommend fluorometry over spectrophotometry. There are no independent fluorometric chlorophyll attenuation coefficients, and each individual fluorometer must be calibrated against spectrophotometric standards. The acid ratio for pure chlorophyll must also be determined for each instrument. Weber et al. (1986) also mention problems of the quenching of chlorophyll a fluorescence by b-carotene and other accessory pigments, an algal species dependent relationship between extract fluorescence and chlorophyll concentration, and the dependence of chlorophyll fluorescence on temperature. High chlorophyll concentrations will quench the fluorescence, thus requiring the dilution of some samples. Marker et al. (1980) also discourage the use of the fluorometric technique in freshwaters if an acidification step is used to determine phaeo-pigments. Apparently the phaeophytin by-product of chlorophyll b has a fluorescence that overlaps significantly with that of phaeophytin a, therefore producing high values for phaeo-pigments. For this last reason, Standard Methods (APHA, 1989) does not recommend the acidification step in inland waters. Total chlorophyll pigments, as discussed above, could be reported.
Recommendations
Based on the theory discussed above and on the methods, observations, and comments by existing programs, the following recommendations are made.
Sampling and Filtration
Samples should be filtered as quickly as possible. In the interim, the water samples should be kept cool and dark. The type of filtration apparatus can be left to the discretion of the coordinator. Providing a complete filtration apparatus has the single advantage of allowing the volunteer to see the color of the filter and thus judge the amount of algae to filter. Its disadvantages are that it is expensive and it requires a considerable amount of manipulation and care by the volunteer. The advantages of using the Swinney filter holder is that it is relatively inexpensive, requires no manipulation by the volunteer, and the filtration is done immediately, therefore requiring no sample bottle or sample preservation. Its disadvantage is that the volunteer does not open the holder, and therefore cannot judge if sufficient sample has been filtered. If the volunteer does use care and dry the filter, there is a greater chance that the filter holder will contain residual water which might promote growth or degradation of the chlorophyll.
Preservation
Keeping the chlorophyll molecule intact until analysis is a concern. Most programs immediately freeze the sample. Freezing seems to provide adequate preservation for several months. Although mailing of the frozen sample to the laboratory is done, experience suggests that unless it can be assured that the sample is delivered within 48 hours, there is a possibility of degradation. Immersing the filter in the solvent or drying the filter are possible alternatives that should be given consideration. The safest method is to have the samples picked up by program personnel.
Analysis
A number of combinations and variations of the chlorophyll technique exist. The important fact is that the final chlorophyll value is highly dependent on the technique used. Without some standardization, program to program comparisons of chlorophyll values should be held as suspect. The concept behind Standard Methods is to provide just that, a standard set of methods for all analysts. The chlorophyll method described in APHA (1989) is recommended. This technique, since it uses acetone as the solvent, probably does not provide total extraction of chlorophyll from some algal cells, but does provide some analytical consistency with historical data. Using a different extractant will undoubtedly change the amount of chlorophyll extracted per unit biomass. It will therefore change any empirical relationships between chlorophyll and other limnological variables such as phosphorus, Secchi depth, etc. Changing extraction solvents should be done only with the knowledge that published empirical relationships may no longer be valid.
The trichromatic equations are not recommended: they take longer and can provide erroneous chlorophyll a values. At best, the chlorophyll a values are equal to those for total chlorophyll pigments. Chlorophyll a values obtained after acidification can be reported but remember that calling the phaeo-pigment corrected value “chlorophyll a” does not make it so. It would be better termed “magnesium-containing pigments.” This value is dependent on the technique and would be expected to vary widely from procedure to procedure.
It is strongly recommended that the total chlorophyll pigment be reported in addition to chlorophyll a. This value, although flawed by interferences by other chlorophylls, phaeo-pigments, as well as a number of other possible interferences, is the only value that remains fairly independent of chlorophyll methodology. Therefore, it is the only measurement that provides historical consistency. Chlorophyll a methodologies have changed over the past 25 years, and with each change, the previous chlorophyll estimates became obsolete and non-comparable to the new methods. If everyone had reported total chlorophyll, at least there would be one consistent value that would allow comparison. In a monitoring program, where historical data consistency is absolutely necessary, this value should be reported.
Literature Cited
American Public Health Association. 1989. Standard Methods for the Examination of Water and Wastewater. 17th Edition. APHA.
Betz, C.R., P.J. Howard, and P.J. Anderson. 1990. Wisconsin’s expanded Self-Help Lake Monitoring Program: results of the 1990 pilot program expansion. Presented at the International Symposium of the North American Lake Management Society.
Betz, C.R. 1992. Lake monitoring Techniques: the next generation. Presented at the 3rd National Citizen Monitoring Conference. Annapolis, MD.
Canfield, D.E. Jr. 1991. Assessment of water quality in the lakes of north and central Florida. Project Completion Report.
Daley, R.J., C.B.J. Gray and S.R. Brown. 1973. A quantitative semiroutine method for determining algal and sedimentary chlorophyll derivates. J. Fish Res. Bd Can. 30: 345-356.
Golterman, H.L. and R.S. Clymo. 1971. Methods for Chemical Analysis of Fresh Waters. IBP Handbook No. 8. Blackwell Scientific.
Hallegraeff, G.M. 1976. Pigment diversity in freshwater phytoplankton. I. A comparison of spectrophotometric and paper chromatographic methods. Int. Revue ges. Hydrobiol. 61: 149-168.
Harvey, H.W. 1934. Amount of phytoplankton population. J. Mar. Biol. Assoc. UK. 19: 761-773.
Herve, S. and P. Heinonen. 1982. Some factors affecting the determination of chlorophyll a in algal samples. Ann. Bot. Fennici. 19: 211-217.
Jeffrey, S.W. and G.R. Humphrey. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanzen Bd. 167: 191-194.
Jones, R.A. and G. F. Lee. 1982. Chlorophyll – a raw water quality parameter. J. AWWA. 90: 490-494.
Lenz, J. and P. Fritsche. 1980. The estimation of chlorophyll a in water samples: a comparative study on retention in a glass-fibre and membrane filter and on the reliability of two storage methods. Arch. Hydrobiol. Beih. Ergebn. Limnol. 14: 46-51.
Lorenzen, C.J. 1967. Determination of chlorophyll and phaeo-pigments: spectrophotometric equations. Limnol. Oceanogr. 12: 343-346.
Marker, A.F.H., E.A. Nusch, H. Rai, and B. Riemann. 1980. The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Arch Hydrobiol. Beih. Ergebn. Limnol. 14: 91-106.
Minnesota Pollution Control Agency. 1989. Water Quality Monitoring for the Clean Water Partnership. A Guidance Document. Minnesota Pollution Control Agency. Division of Water Quality.
Odum, H.T., McConnell, W. and Abbott, W. 1958. The chlorophyll a of communities. Publ. Inst. Mar. Sci., Texas 5:65-96.
Parsons, T.T. and J.D.H. Strickland. 1963. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. J. Mar. Res. 21: 155-163.
Phinney, D.A. and C.S. Yentsch. 1985. A novel phytoplankton chlorophyll technique: toward automated analysis. J. Plankton Res. 7: 633-642.
Prepas, E.E., M.E. Dunnigan, and A.M. Timbee. 1988. Comparison of in situ estimates of chlorophyll a obtained with Whatman GF/F and GF/C glass-fiber filters in mesotrophic to hypereutrophic lakes. Can. J. Fish. Aquat. Sci. 45: 910-914.
Richards, F.A. and Thompson, T.F. 1952. The estimation and characterization of plankton populations by pigment analyses. II. A spectrophotometric method for the estimation of plankton pigments. J. Mar. Res. 11:156-172.
Sartory, D.P. and J.U. Grobbelaar. 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114: 177-187.
UNESCO. 1966. Monograph on oceanographic methodology. I. Determination of photosynthetic pigments in sea water. United Nations Educ., Sci., and Cult. Org., Paris.
Weber, C.I., L.A. Fay, G.B. Collins, D.E. Rathke, and J. Tobin. 1986. A review of methods for the analysis of chlorophyll in periphyton and plankton of marine and freshwater systems. Ohio State University Sea Grant Program Tech Bull. OHSU-TB-15.